Trusted by Industry Leaders
Our solutions are trusted by some of the most respected companies in the industry
Capture. Secure
Integrate. Comply.
Logilab SDMS is a next-generation Scientific Data Management System (SDMS) built for regulated labs that demand AI-ready, cloud-native scientific data management
Designed to work with any instrument — from modern HPLC and LC-MS to legacy RS-232 devices — Logilab SDMS ensures your laboratory data is secure, compliant, vendor-neutral, and instantly accessible
Cost Savings in paper & reporting
Faster Data Retrieval
Fewer
Transcription Errors
Faster Audit Prep
Why Modern Labs choose Logilab SDMS
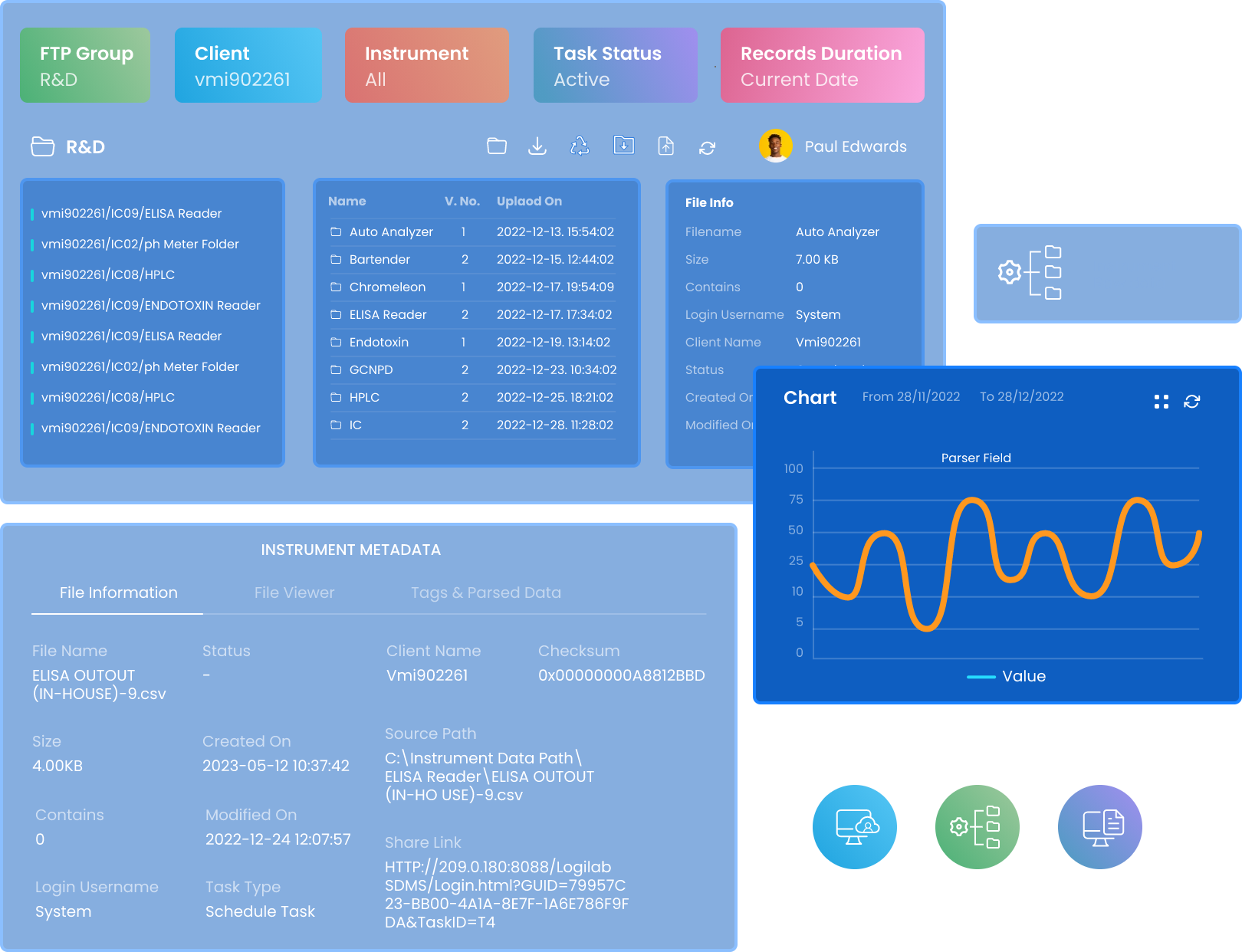
Automated, Multi- Instrument Data Capture
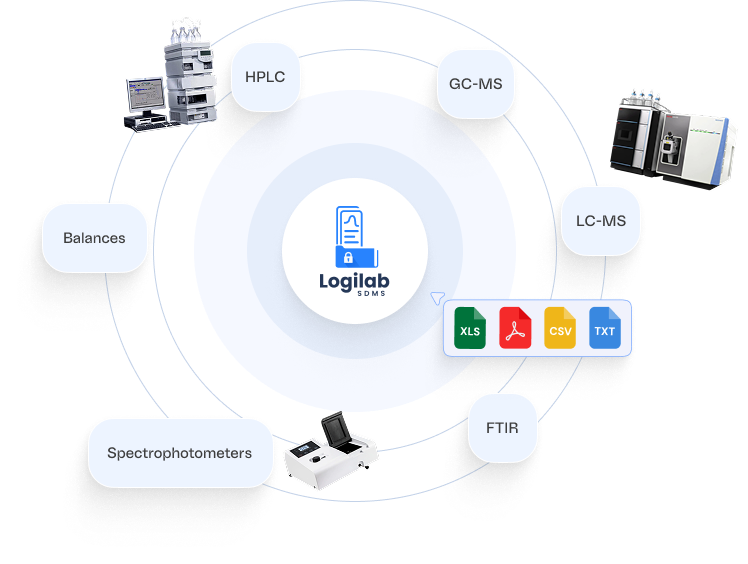
- Ingest raw and processed data directly from HPLC,GC-MS,LC-MS,FTIR, spectrophotometers, balances, and other instruments.
- Compatible with leading brands including Agilent,Thermo Fisher Scientific, Shimadzu, and Waters
- Supports PC-based (File) & Port–based systems, RS-232, and TCP/IP protocols for flexible connectivity
- Built as a vendor-neutral instrument integration platform, enabling data capture across heterogeneous lab environments
AI-Driving Parsing Engine & Metadata Tagging
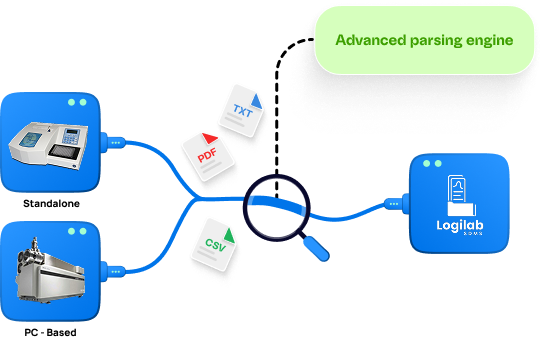
Advanced parsing engine automatically extracts critical parameters, applying smart metadata tagging for projects, samples, batches & more

Supports smart FAIR data principles (Findable, Accessible, Interoperable, Reusable) for improved scientific data reuse and collaboration
Enables predictive insights from laboratory data and provides structured data for
Compliance
You Can
Trust
Built for 21 CFR Part 11 Compliance
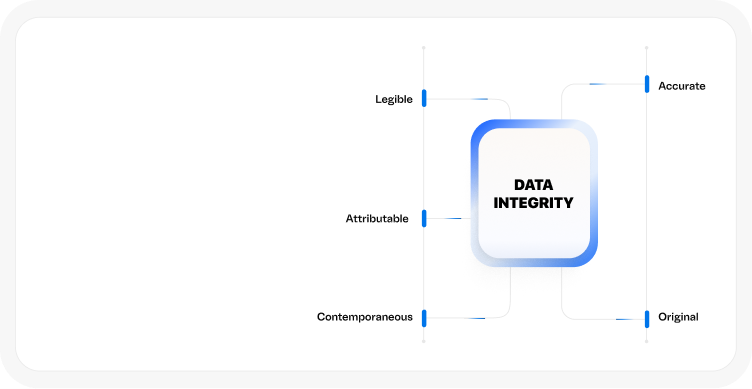
GLP, GAMP 5, ALCOA+ data integrity principles, and EudraLex Annex 11 standards.

IQ/OQ/PQ validation
covering regulatory alignment, IQ/OQ/PQ validation, and audit-readiness.


GLP, GAMP 5, ALCOA+ data integrity principles, and EudraLex Annex 11standards.

Ideal for GxP-regulated labs across pharmaceuticals, biotechnology, CROs, and more.
Includes FDA audit-ready features : secure electronic signatures, comprehensive audit trails, and role-based access control.
covering regulatory alignment, IQ/OQ/PQ validation, and audit-readiness.
Full details available in our
Seamless Integration with ELN, LIMS, MES, CDS, and ERP Systems
Logilab SDMS connects effortlessly with a wide range of lab informatics and enterprise
platforms to ensure smooth, automated data flow across your operations.
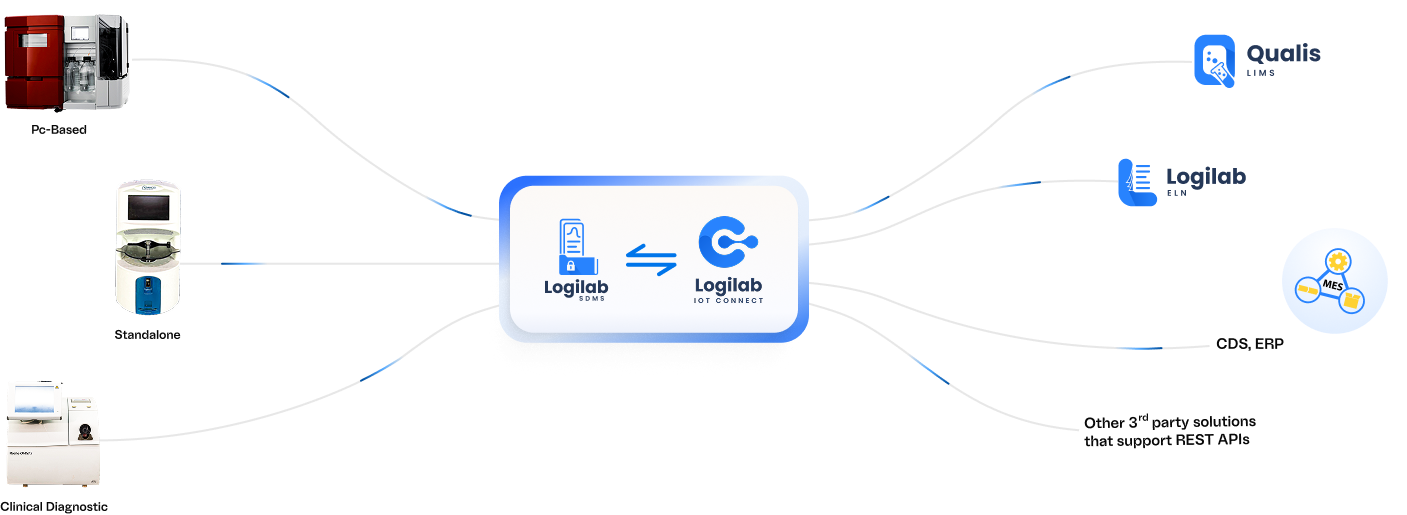
- Out-of-the-Box (OOTB) Integration with Logilab ELN & Qualis LIMS
Built-in, seamless connectivity with Logilab ELN & Qualis LIMS enables direct data flow from instruments into experimental templates, maintaining end-to-end sample and result traceability.
- Manufacturing Execution Systems (MES)
Integration with Pasex MES supports in-process quality control by feeding critical results back to production in real time
- Enterprise Resource Planning (ERP)
Interfaces with SAP and other ERP systems for batch release, quality control, and compliance reporting
- Electronic Lab Notebooks (ELNs)
Supports integration with Revitty ELN and PerkinElmer Signals ELN allowing instrument-generated data to be automatically populated into experiment records, eliminating manual transcription
- Chromatography Data Systems (CDS)
Capture and archive results from Chromeleon and other CDS platforms, complete with metadata tagging
- Microsoft Dynamics 365
Supports integration with Microsoft Dynamics 365 Platform for Warehouse Inventory Management
Cloud-Enabled Deployment Options
- Deploy Logilab SDMS securely on AWS, Microsoft Azure, or customer-owned cloud/On-premise environments, giving you full control over infrastructure and compliance alignment
- Benefit from scalable storage, high availability, and globally accessible data — all while meeting regional data residency requirements
- Choose between public, private, or hybrid cloud setups based on your IT and regulatory needs.



CFR Gateway for
Legacy Instrument Compliance
- Brings non-compliant or older instruments under 21 CFR Part 11 governance
- Tracks instrument use from boot-up to data generation
- Protects against unauthorized access or data tampering
Key Benefits at a Glance
| Feature | Benefit | Compliance Tie-In |
|---|---|---|
 Automated Data Capture Automated Data Capture |
No manual transcription errors | ALCOA+, ISO 17025 |
 AI Powered Parsing AI Powered Parsing |
Smart metadata tagging & searchability | FAIR data principles |
 Audit Trails Audit Trails |
Complete activity logs | 21 CFR Part 11, GAMP 5, EudraLex Annex 11 |
 Integration Suite Integration Suite |
Connects to ELN, LIMS, MES, ERP | Seamless data transfer for Data Integrity protection |
 Cloud Deployment Cloud Deployment |
Accessible anywhere, secure | Regional data residency compliance |
 CFR Gateway CFR Gateway |
Brings legacy instruments into compliance | FDA audit-ready |
Customer Success Snapshot

Our implementation was a great SUCCESS! For our digital journey with respect to automation with highest order of data integrity & compliance.
Roland Smith -VP Digital Solutions

Our implementation was a great SUCCESS! For our digital journey with respect to automation with highest order of data integrity & compliance.
Start Your Digital Transformation
Future-proof your laboratory with Logilab SDMS — the AI-ready, vendor-neutral, cloud-native Scientific Data Management System trusted by labs worldwide.